|
Leclanché Cells are the carbon-zinc primary
batteries which have been largely replaced by alkaline cells.
Anode: Zinc
Cathode: Manganese Dioxide (MnO2)
Electrolyte: Ammonium chloride or zinc chloride
dissolved in water
Applications: Flashlights, toys, moderate drain
use
The basic design of the Leclanché cell has been
around since the 1860s, and until World War II, was the only one in wide use.
It is still the most commonly used of all primary battery designs because of
its low cost, availability, and applicability in various situations. However,
because the Leclanché cell must be discharged intermittently for best
capacity, much of battery research in the last three decades has focused on
zinc-chloride cell systems, which have been found to perform better than the
Leclanché under heavier drain.
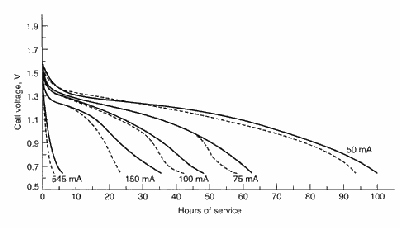
This figure shows typical discharge curves for
general-purpose Leclanché zinc chloride D-size cells discharge 2 h/day
at 20º C. Solid line—zinc chloride; broken
line—Leclanché (Linden 8.18). The zinc-chloride cell has a
higher service life and voltage than the Leclanché (at both higher and
lower discharge rates).
In an ordinary Leclanché cell the electrolyte
consists (in percent of atomic weight) of 26% NH4Cl (ammonium
chloride), 8.8% ZnCl2 (zinc chloride), and 65.2% water. The overall
cell reaction can be expressed:
Zn + 2MnO2 +2NH4Cl —> 2MnOOH
+ Zn(NH3)2Cl2 E=1.26
The electrolyte in a typical zinc chloride cell consists
of 15-40% ZnCl2 and 60-85% water, sometimes with a small amount of
NH4Cl for optimal performance. The overall cell reaction of the zinc
chloride as the electrolyte can be expressed:
Zn + 2MnO2 + 2H2O + ZnCl2
—> 2MnOOH + 2Zn(OH)Cl
MnO2, is only slightly conductive, so graphite
is added to improve conductivity. The cell voltage increases by using
synthetically produced manganese dioxide instead of that found naturally
(called pyrolusite). This does drive the cost up a bit, but it is still
inexpensive and environmentally friendly, making it a popular cathode.
These cells are the cheapest ones in wide use, but they
also have the lowest energy density and perform poorly under high-current
applications. In fact some pyrolusite MnO2 deposits are pure enought to be used
directly after milling. The zinc carbon design is reliable and more than
adequate for many everyday applications. |
