
CHAPTER 6
WHAT TAKES PLACE DURING CHARGE
Voltage. Starting with a battery which has been discharged until its voltage has decreased to 1.7 per cell, we pass a current through it and cause the voltage to rise steadily. Fig. 24 shows the changes in voltage during charge. Ordinarily the voltage begins to rise immediately and uniformly. If, however, the battery has been left in a discharged condition for some time, or has been "over discharged," the voltage rises very rapidly for a fraction of the first minute of charge and then drops rapidly to the normal value and thereafter begins to rise steadily to the end of the charge. This rise at the beginning of the charge is due to the fact that the density of the acid in the pores of the plates rises rapidly at first, the acid thus formed being prevented from diffusing into the surrounding electrolyte by the coating of sulphate. As soon as this sulphate is broken through, diffusion takes place and the voltage drops.
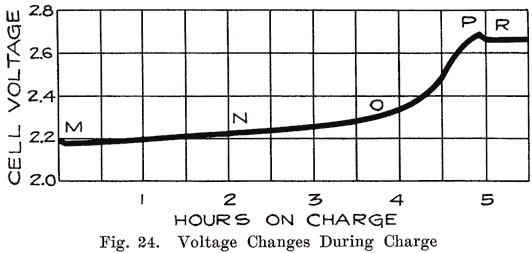
As shown in Fig. 24, the voltage remains almost constant between the points M and N. At N the voltage begins to rise because the charging chemical reactions are taking place farther and farther in the inside parts of the plate, and the concentrated acid formed by the chemical actions in the plates is diffusing into the main electrolyte. This increases the battery voltage and requires a higher charging voltage.At the point marked 0, the voltage begins to rise very rapidly. This is due to the fact that the amount of lead sulphate in the plates is decreasing very rapidly, allowing the battery voltage to rise and thus increasing the charging voltage. Bubbles of gas are now rising through the electrolyte.
At P, the last portions of lead sulphate are removed, acid is no longer being formed, and hydrogen and oxygen gas are formed rapidly. The gas forces the last of the concentrated acid out of the plates and in fact, equalizes the acid concentration throughout the whole cell. Thus no further changes can take place, and the voltage becomes constant at R at a voltage of 2.5 to 2.7.
Density of Electrolyte. Discharge should be stopped when the density of the electrolyte, as measured with a hydrometer, is 1.150. When we pass a charging current through the battery, acid is produced by the chemical actions which take place in the plates. This gradually diffuses with the main electrolyte and causes the hydrometer to show a higher density than before. This increase in density continues steadily until the battery begins to "gas" freely.
The progress of the charge is generally determined by the density of the electrolyte. For this purpose in automobile batteries, a hydrometer is placed in a glass syringe having a short length of rubber tubing at one end, and a large rubber bulb at the other. The rubber tube is inserted in the cell and enough electrolyte drawn up into the syringe to float the hydrometer so as to be able to obtain a reading. This subject will be treated more fully in a later chapter.
Changes at Negative Plate. The charging current changes lead sulphate into spongy lead, and acid is formed. The acid is mixed :with the diluted electrolyte outside of the plates. As the charging proceeds the active material shrinks or contracts, and the weight of the plate actually decreases on account of the difference between the weight and volume of the lead sulphate and spongy lead. If the cell has had only a normal discharge and the charge is begun soon after the discharge ended, the charge will proceed quickly and without an excessive rise in temperature. If, however, the cell has been discharged too far, or has been in a discharged- condition for some time, the lead sulphate will not be in a finely divided state as it should be, but will be hard and tough and will have formed an insulating coating over the active material, causing the charging voltage to be high, and the charge will proceed slowly. When most of the lead sulphate has been reduced to spongy lead, the charging current will be greater than is needed to carry on the chemical actions, and will simply decompose the water into hydrogen and oxygen, and the cell "gasses. " Spongy lead is rather tough and coherent, it, and the 'bubbles of gas which form in the pores of the negative plate near the end of the charge force their way to the surface without dislodging any of the active material.
Changes at the Positive Plate. When a cell has been discharged, a portion of the lead peroxide has been changed to lead sulphate, which has lodged in the pores of the active material and on its surface. During charge, the lead combines with oxygen from the water to form lead peroxide, and acid is formed. This acid diffuses into the electrolyte as fast as the amount of sulphate will permit. If the discharge has been carried so far that a considerable amount of sulphate has formed in the pores and on the surface of the plate, the action proceeds very slowly, and unless a moderate charging current is used, gassing begins before the charge is complete, simply because the sulphate cannot absorb the current. The gas bubbles which originate in the interior of the plate force their way to the surface, and in so doing cause numerous fine particles of active material to break off and fall to the bottom of the jar. This happens because the lead peroxide is a granular, non-coherent substance, with the particles held together very loosely, and the gas breaks off a considerable amount of active material.
|
|
|
| |||||||||