
| July 31, 2019 | ||||||||
Capacity loss during pulse discharge of batteriesMost battery discharge curves show constant-current or constant-power discharge. Batteries that have a significant Peukart effect exhibit lower capacity at higher discharge currents. Most primary cells, and lead acid secondary cells show significant Peukart effect. For example, the following curve shows how a lithium manganese oxide primary cell loses capacity under higher discharge conditions. |
|
|||||||
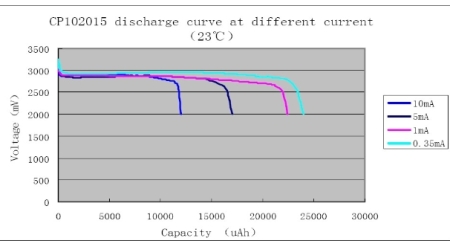 In like manner a lead acid battery will typically discharge in half an hour when it is discharged at the nominal one hour rate, or the 1C rate, where the capacity C is measured over a 20 hours discharge. Following is a curve of the equivalent capacity of a 55AH sealed lead acid battery when discharged at rates from 222A to 2.75A. 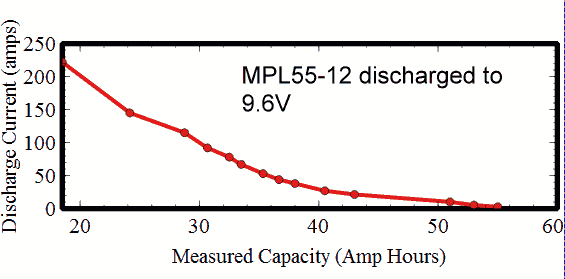 Lithium-ion and NiCad batteries have a low Peukart effect, and so high discharge rates don't reduce the capacity very much. But an intermediate case is of great interest. What would happen if you discharged a battery in high-current pulses spaced far apart? Would you get a low capacity associated with the high instantaneous discharge rate, or a high capacity based on a low average discharge rate? The answer depends on what is causing the battery to lose capacity when discharged quickly. Resistance losses internal to the battery will increase as a proportion of the load resistance as the current goes up. As the current goes up the resistance of the load must go down, and the total internal resistance of the battery will go up, due to factors such as polarization. Heating in the battery may also cause the internal resistance to go down as the ionic mobilities improve. Resistive losses in the battery will not be recoverable, since they are incurred at the time of discharge. Polarization of the battery can also cause the capacity to go down at high discharge currents, but capacity loss due to polarization may be recoverable. Letting the battery rest between pulses, or reducing the current drawn significantly between pulses, can allow the battery chemistry to relax and increase the capacity of the battery over that of a continuous high current discharge. So polarization losses can be recoverable. In cases where the majority of the discharge is at low rates, with a few high current pulses spaced widely, the capacity of the battery will be dominated by the low rate. The maximum power is obtained in a pulse when the load resistance is equal to the internal battery resistance. In this case the discharge voltage will be half of the open circuit voltage. The result is the maximum possible power dissipated in the load, but it also means that half of the power is dissipated in the cell. Capacity is also decreased at low temperature, and increased at high temperature. The following curve shows that this lithium manganese oxide primary cell loses capacity if continuously discharged at low temperatures. 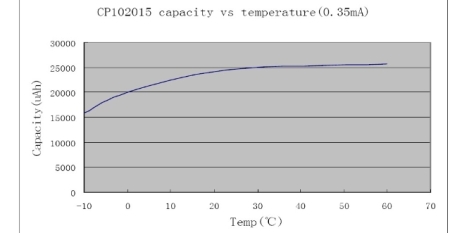 This capacity loss is also somewhat reverseable when the battery is warmed. Of course the energy lost during the discharge due to increased resistance is lost forever, but polarization due to the discharge at low temperatures can be recovered. I don't have a rule of thumb approximating how a battery will lose capacity under high current discharge pulses. Every scenario deserves its own testing to estimate the expected battery life. But several items can be noted. 1. The capacity of the battery for a low duty cycle of high current pulses will be according to the average discharge rate, rather than the high discharge rate. 2. If the pulse rate and duty cycle are not such that the battery can recover between pulses the battery life will be reduced compared with its average rate discharge curve. 3. At the end of life the battery will easily support a small continuous current, but may fail to supply the pulsed current at a voltage necessary for the application. 4. Different cells from the same batch may act differently, so don't depend on testing a single cell. |
||||||||
|
|
|
| |||||||||